
Three bioequivalence clinical trials were performed comparing Repinex® XL 2mg prolonged-release tablets with Requip® XL (ropinirole) 2mg (GlaxoSmithKline, Munich, Germany) and one bioequivalence study was performed comparing Repinex XL 8mg prolonged-release tablets and Requip XL 8mg.1
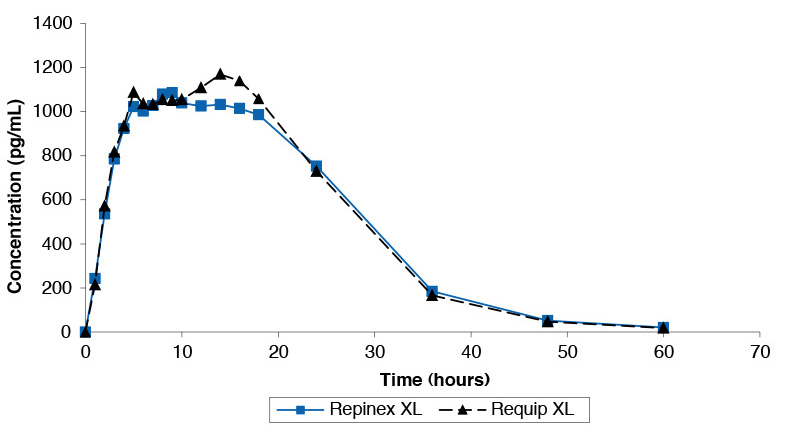
Single dose study comparing Repinex XL 2mg and Requip XL 2mg under fasted conditions
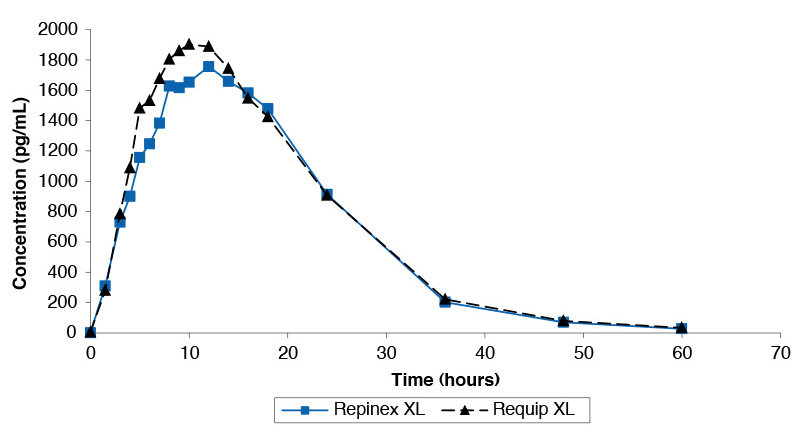
Single dose study comparing Repinex XL 2mg and Requip XL 2mg under fed conditions
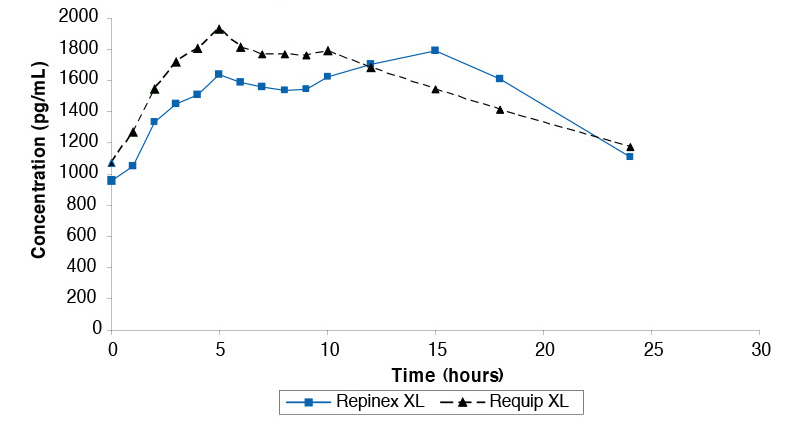
Steady state study comparing Repinex XL 2mg and Requip XL 2mg under fasted conditions
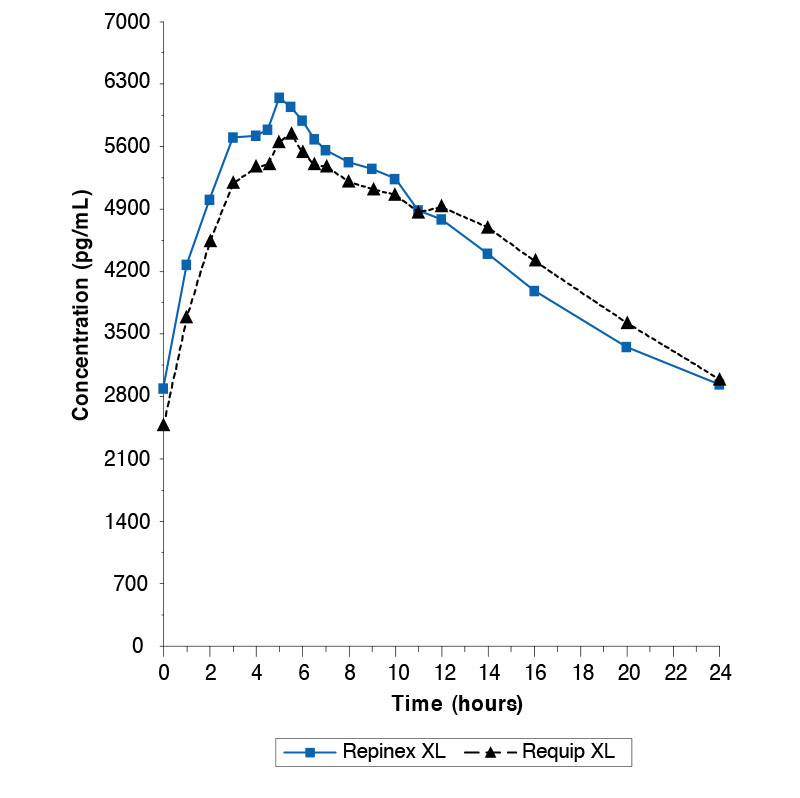
Single dose study comparing Repinex XL 8mg and Requip XL 8mg under fasted conditions
References: 1) Data on file. 1010067149 v 6.0 September 2023.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to Aspire Pharma Ltd on 01730 231148
For more information about Repinex XL, please click here for the prescribing information.














